Transcranial Magnetic Stimulation (TMS) is a non-invasive stimulation procedure that uses transient electromagnetic fields to act in a focused manner on a particular brain region. It can be used as a therapy for depression, Parkinson’s disease or obsessive-compulsive disorders in patients resistant to drug treatments. Researchers from the Paris Brain Institute also showed lasting effects of TMS in rehabilitation after a stroke.
Recording of intraneuronal electrical activity before and after TMS
Therapeutic effects of TMS in humans
It is generally considered that high intensity magnetic stimulation used in humans generate an electric field sufficient to modulate the activity of neurons: triggering nerve impulses encoding cerebral information and inducing plasticity phenomena in the neuronal networks of the targeted region.
However, although its therapeutic efficiency has been recognized in a number of cases, the mechanisms that allow TMS to restore or durably modify neuronal activities in the stimulated brain regions remain poorly understood.
TMS at the neuronal level
For the first time, Manon Boyer and Séverine Mahon, respectively doctoral student and researcher in the team “DYNAMICS OF EPILEPTIC NETWORKS AND NEURONAL EXCITABILITY” in collaboration with Dr Antoni Valero-cabre Team “FRONTLAB: FRONTAL FUNCTIONS AND PATHOLOGY” (institutducerveau-icm.org) at the Brain Institute, have directly demonstrated the effect of low-intensity TMS on the excitability and spontaneous activity of neurons in the cerebral cortex, activity that allows these cells to communicate with each other and to transmit information to peripheral organs and muscles. This work is published in The Journal of Physiology.
The researchers combined the application of repeated low-intensity magnetic stimulation to the somatosensory cortex of an experimental model with intracellular recording of the electrical activity of the underlying neurons; a technique allowing to understand the effect of TMS seen from inside the neurons.
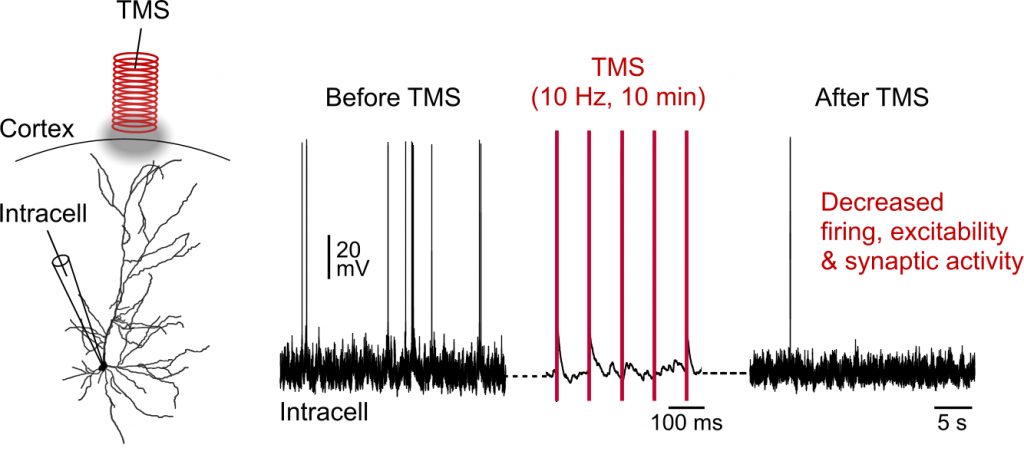
Recording of intraneuronal electrical activity before and after TMS
They showed that repetitive low intensity TMS can also trigger action potentials in neurons and induce plasticity expressed by a lasting decrease in the spontaneous activity of neurons, in their excitability and in the amplitude of synaptic events (reflecting a decrease in the transmission of information between neurons). These changes are not reproduced by directly stimulating the cells with an electric current of the same intensity, directly demonstrating the specific role of the magnetic field.
New therapeutic avenues for TMS
In conclusion, this work shows that low-intensity TMS has lasting effects on the excitability and discharge activity of neurons in the cortex and opens up new therapeutic avenues for pathologies in which cerebral hyperexcitability is observed, such as epilepsy or Tourette’s syndrome. Indeed, in epilepsy, we observe an abnormally high electrical activity of a set of neurons in the cerebral cortex which then form an epileptic focus.
This work shows that low-intensity TMS, a non-invasive therapy that can be easily deployed at the patient’s bedside, is a very promising approach for patients with epilepsy.










