In 2023, the team led by Professor Fanny Mochel (AP-HP, Sorbonne University), a Paris brain Institute researcher, showed that daily dose of leriglitazone slow down the progression of myelopathy in patients with X-linked adrenoleukodystrophy, and above all, reduce their risk to develop an acute cerebral form (CALD) of the disease. This year, in scientific paper published in Brain, the same team has shown that leriglitazone can halt the progression of CALD.
The cerebral form of X-linked adrenoleukodystrophy (CALD) is an aggressive inflammation of the brain resulting in cerebral demyelination and rapid cognitive and motor decline, with a median survival of 3 years. CALD develops in a third of boys and over half of men with X-linked adrenoleukodystrophy, the most common hereditary white matter disease.
While transplantation of hematopoietic stem cells – the cells that generate the brain’s immune cells – is the gold standard in the early stages of the disease, many male patients are ineligible because of their age, the lack of a suitable donor or the presence of lesions in the corticospinal tract.
A molecule with multiple effects
Leriglitazone, developed by the Spanish biotech Minoryx Therapeutics, present the advantage to cross the blood-brain barrier, the protective membrane of the brain which often constitute an obstacle for neurological and psychiatric diseases treatment.
This molecule also has the ability to protect oligodendrocytes, the cells that produce myelin, to reduce the level of pro-inflammatory cytokines and thus inflammation, and to preserve neurons.
A non-invasive alternative therapeutic solution
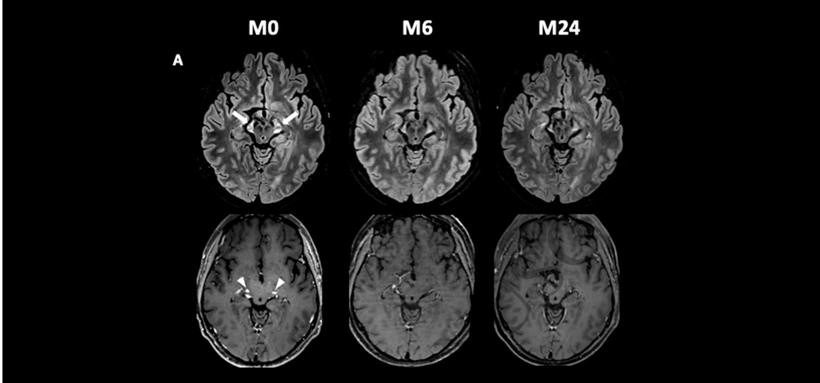
In a therapeutic trial coordinated by Prof. Fanny MOCHEL, 13 CALD patients aged between 19 and 67 were treated orally with leriglitazone.
Each participant underwent motor and cognitive neurological examinations, magnetic resonance imaging (MRI) and blood tests every 3 months over a 24-month period.