New study conducted by Irini Kessissoglou in the “Brain development” team headed by Bassem Hassan at Paris Brain Institute reveals new functions for the Amyloid Precursor Protein (APP) homologue in drosophila. Results are published in PLOS Biology.
The amyloid precursor protein (APP) is an essential protein, best known for its involvement in the pathway at the origin of amyloid beta, the component of the main lesion in Alzheimer’s disease (AD), the amyloid plaques. Mutations in the APP gene are linked with early-onset familial cases of AD. However, the physiological role of APP in adult brain function and whether there is any link between this normal role and defects seen in AD remains unclear. To address this issue, Kessissoglou et al. investigated the function of this protein in the fruit fly, Drosophila melanogaster, a model organism often used in biomedical research which possesses a homologue of APP, called APPL (Amyloid precursor protein like).
Neurons, like all cells, possess a system called the endolysosomal recycling and degradation pathway for separating, recycling and, when necessary, trashing its proteins. This system guarantees protein and organelles balance neurons and it has been shown to be defective in human neurons with AD patient mutations, suggesting that APP may be be important for this process under normal conditions.
Using Drosophila model of APPL loss of function, the authors explored the molecular and cellular mechanisms underlying the brain homeostasis, which can be defined as the physiological processes necessary to maintain brain health and restoring balance in case of injury or diseases.
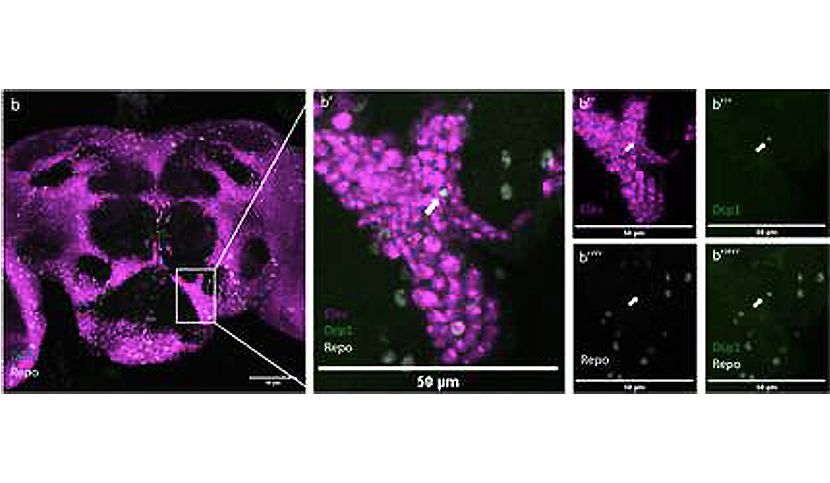
They identified a pathway crucial for adult brain homeostasis involving APPL. Indeed, APPL loss of function leads in a disruption of the endolysosomal function in neurons followed by cell death. The dead neuronal cell, which are significantly increased with the loss of APPL, accumulate in the brain at early age. Moreover, not only did flies lacking APPL have a shorter lifespan and neurodegeneration by 30 days old (a middle-old age at the scale of a Drosophila), their brain also showed signs of dysfunctional homeostasis as early as 7 days old.
One key point of the study is the evidence that the extracellular domain of APPL secreted by neurons interacts with glial cells to regulate their endolysosomal pathway and enable the clearance of neuronal debris. Glial cells are key elements of brain homeostasis as they are involved in immune response, providing nutrients to neurons and clearance of cellular wastes. Overall, this suggest that APPL is part of a neuro-glial signaling system responsible for monitoring brain health.
The results of this study bring new insights on the role of APP in a physiological context and highlight its importance for adult brain homeostasis. The findings regarding the consequences of the loss of APP suggests a strong link between its physiological functions and the defects observed in familial cases of Alzheimer’s disease. The early effects observed in flies lacking APPL support the idea of long-term modifications in the brain occurring before the onset of clinical symptoms, fostering new research on early endosomes and neuro-glial interactions in this disease.
Source
Kessissoglou IA, Langui D, Hasan A, Maral M, Dutta SB, Hiesinger PR, Hassan BA.PLoS Biol. 2020 Dec 8