From the very first weeks of life, countless connections are forged between neurons to ensure the propagation of nerve signals. These connections gradually shape the final architecture of the brain, known as the connectome. Our ability to perform complex cognitive tasks, such as spatial orientation or problem-solving, hinges on its structure… But how does it emerge during development? At Paris Brain Institute, Vito Dichio and Fabrizio de Vico Fallani have introduced a computer model that replicates the brain wiring trajectory in the C. elegans worm. Eventually, they hope to apply this new model, described in Physical Review Letters, to other species, including humans.
In organisms with a nervous system, that is, most animal species, the connectome—the network formed by all the connections that link neurons to each other—is a crucial element for the proper functioning of the brain. It is essential for integrating information from the environment and for the emergence of primary cognitive functions, such as attention or memory. When it is damaged due to an injury—after a stroke, for example —brain function is also affected.
“The connectome map varies across species, which have more or fewer neurons, and individuals. It can also reorganise itself throughout life to compensate for certain deficits: this capacity is called brain plasticity,” Fabrizio de Vico Fallani (Inria), leading the NERV team at Paris Brain Institute, explains.
However, the formation of neural connections during brain development is not entirely random. It follows genetically encoded rules and is guided by selection: the body retains the connections that allow the brain to function correctly. The team is trying to mathematically describe these organisational rules—the product of millions of years of evolution—and simulate them in a model of brain development.
Finding the laws of brain wiring
“We use several model organisms to study the connectome, such as the nematode and the Drosophila. These species are extremely useful: they have a small number of neurons— between tens and hundreds of thousands—which allow us to draw an exact map of their neural connections at different stages of development,” Vito Dichio, a former PhD student in the team and first author of the paper, says. “In this study, we used the connectome of the C. elegans worm,whose brain has about 180 neurons.”
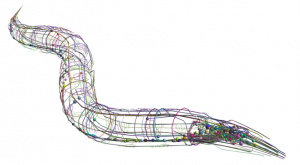
C. elegans connectome. Crédit : OpenWorm project.
To simulate the worm’s brain development, the researchers used a model based on the “exploration-exploitation” dynamics, i.e. a combination of random exploration of new neuronal connections and exploitation of the most viable connections to support cognitive function. After defining the end state of the adult connectome in the worm—i.e. the ideal neuronal characteristics of an animal able to move, feed and reproduce—they let their algorithm “search” for the developmental trajectory that would lead, step by step, to this result.
“In each simulation, the development trajectory of our virtual nematode was slightly different,” Vito Dichio explains. “However, on average, the neural network formation reproduced the same intermediate stages biologically observed in the worm’s brain development. For us, this is further proof that the wiring of the brain follows very simple mathematical rules, which are nonetheless at the root of the extraordinary complexity of the nervous system”. But are these simple rules universal? “To find out, we’ll have to test this model on the connectome of other species, such as flies, mice and zebrafish, and eventually on humans“, the researcher adds.
Predicting the optimal development trajectory
Indeed, if we ever have a complete database of the human connectome, and if this model stands the test of successive tests in different species, it could help researchers define the optimal developmental trajectory for a healthy brain. “We’re still a long way off! However, we may one day be able to pinpoint the exact moment when development deviates from this ideal path, giving rise to disorders at the root of neurodegenerative diseases,” Fabrizio De Vico Fallani says. “This could help us to intervene in patients at the right time with neuroprotective drugs.”
In the near future, the model could also be applied to brain plasticity to predict patients’ recovery from stroke and guide it through interventional techniques, such as deep brain stimulation.
“Beyond these important applications, we hope to contribute to a better understanding of brain maturation. It’s a process whose complexity seemed irreducible just ten years ago but whose principles we are increasingly grasping—not least thanks to the contribution of disciplines that have long seemed irrelevant to describing the organisation of living beings, such as mathematics”, the researcher concludes.
FUNDING
This study was funded by a European Research Council (ERC) grant.
Reference
Dichio, V., De Vico Fallani, F. Exploration-exploitation paradigm for networked biological systems. Physical Review Letters, March 2024.
DOI : 10.1103/PhysRevLett.132.098402.