Project summary
HomTasBeh is a European research and training project conducted by postdoctoral fellow Liqun Yuan, who has been recruited at ICM thanks to the funding of the Marie Skłodowska-Curie Individual Fellowship programme.
Within HomTasBeh, three aims are carried out in parallel, and each has the potential to create novel insight into the gustatory biology. More importantly, the combination of these three aims will shed the light on the relationship between neuronal circuit anatomy, activity, molecular features and animal behaviour.
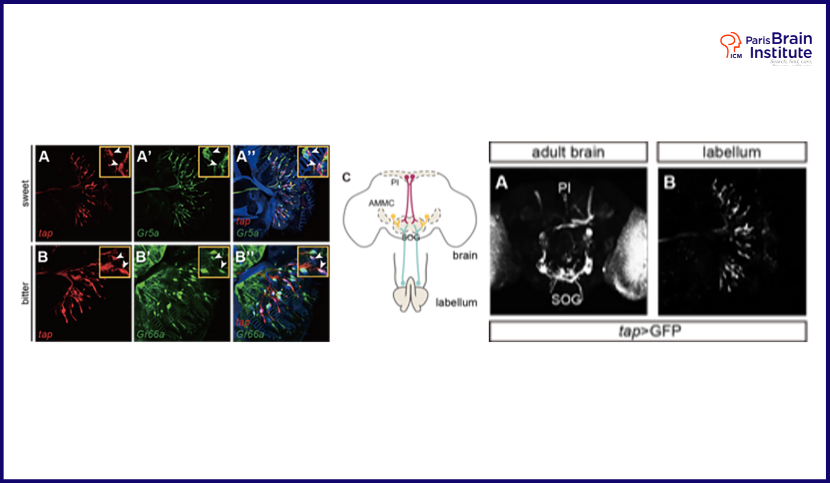
- Identify the wiring diagram of the Tap Gustatory Circuit (TGC).
In Drosophila, a mini circuit of sweet perception has been recently identified. For other modalities, almost all the projection and regulatory neurons are unknown. Tap positive cells have been confirmed to overlap with subsets of sweet and bitter sensory neurons, as well as a few previously undescribed gustatory sensory neurons. Therefore, firstly I am examining which taste stimuli these Tap+ neurons respond to, aiming to investigate the external trigger of the TGC. Additionally, I identified Tap positive neurons in novel cell populations within the region where known gustatory regulatory, and putative projection neurons arborize. Thus, I am studying whether these sensory, regulatory and projection neurons directly communicate with each other, responding to similar taste stimuli, to form an entire neuronal circuit.
- Define the relationship between TGC activity, feeding behaviour and energy homeostasis.
The gustatory network assembles and exchanges information according to internal and external status to direct a behavioural decision. However, it is not clear how gustatory neuronal circuit responds to energy stasis. I am characterizing neuronal activity, behaviour and metabolism in holistic multi-modal approach. Specifically, the correlation among TGC, food intake, body weight, fly rhythm and activity are investigated to reveal the strategy of the animals in responding to both external and internal changes.
- Characterize Tap function within the TGC.
In parallel with the previous aim, I will study the function of Tap in the TGC. I found that Tap expression changes in the gustatory regulatory neurons upon starvation, suggesting that Tap levels are regulated by internal satiety state. I will study Tap function in regulating energy stasis to circuit activity to help generate state-appropriate behaviours. Thus, Tap function will be characterized by gain and loss of function approaches to uncover the molecular mechanism of such correlations.
Results
We observed Tap in several neuronal populations in different levels of the feeding circuit, as well as the gut. Although the physiological study of feeding behaviour in Tap mutant is relatively preliminary, the present data suggests that ectopic expression of Tap in all the Tap neurons reinforces the total food consumption of flies, accompanied with reduced sleep duration and more active locomotion. However, it seems knocking down Tap in either Tap neurons or sugar sensory neurons does not impair the sensitivity of sweet taste in the flies. This implies Tap regulates the feeding circuit via some subprograms other than meal initiation. It is possible that Tap acts as a global modulator of the feeding circuit via buffering the energy homeostasis.
The perception of sweet and bitter taste and the regulation of these two sensory pathways are believed to be independent. Firstly, they detect the stimuli via separate populations of gustatory sensory neurons (GSNs); and these neurons present converse information to the proboscis such that sugar GSNs send “attractive” signal, whereas bitter GSNs send “aversive” signal when they are artificially activated in the absence of taste. Secondly, the projection and intern neurons identified till now are only sweet specifically, suggesting that bitter and sweet tastes utilize independent intern neurons to transmit the taste information. Finally, even though certain states regulate sweet and bitter perception at the same time, such as huger which enhances sensitivity to sugar and inhibits bitter sensitivity, no neuromodulation cross talk between these two sensory pathways has been observed. All the present studies suggest sweet and bitter sensory pathways are independent and reciprocal.
In a natural environment, however, food resources always contain a mixture of taste modalities. There must be some circuit mechanisms for higher order brain circuits to regulate feeding behaviours in response to complex mixtures of tastants in the food, such as whether to accept or reject the food, or take some of the food and disengage in favour of foraging for more nutrient-rich food. It is very interesting that Tap, with a very restricted expression pattern, is expressed in both sweet and bitter GSNs. It is reasonable to speculate that Tap functions in the integration of different taste information and the regulation of behavioural decisions. Therefore, it is worth studying the activations of both sweet and bitter GSNs as well as the expression of neuromodulators upon manipulation of Tap levels and/or activity.
Contact
For further information or collaboration, please contact the fellow, Liqun YUAN: liqun.yuan@icm-institute.org
 This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 797014.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 797014.
The project results reflect the authors view, the Agency and the Commission are not responsible for any use that may be made of the information.